Alpha-hydroxy-beta-triazolo-tetrazole for Click-chemistry
Référence
08758-01
Mots-clés
Statut des brevets
European priority patent application filed on March 11th, 2016 and entitled “ALPHA-HYDROXY-BETA-TRIAZOLO-TETRAZOLES”
US priority patent application filed on March 11th, 2016 and entitled “ALPHA-HYDROXY-BETA-TRIAZOLO-TETRAZOLES”
Inventeurs
François COUTY; Bruno DROUILLAT; Pierre QUINODOZ; Karen WRIGHT
Statut commercial
Exclusive or non-exclusive license
Collaboration
Laboratoire
Institut Lavoisier de Versailles (ILV)
http://www.ilv.uvsq.fr
UMR 8180, Versailles, France
Description
Context
Click chemistry consists in synthezing chemicals via the assembly of smaller modular units. The invention relates more precisely to the CuAAC reaction ; copper(I)-catalysed azide-alkyne cycloaddition.
Technical description
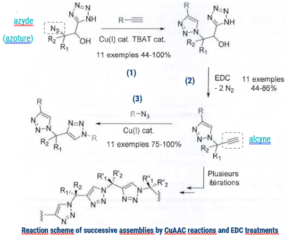
The invention lies in the use of new compounds (alpha-hydroxy-beta tetrazoles) in click chemistry, allowing two ligations thanks to two successive CuAAC reactions.
The first ligation is made with molecule (1), then the alpha-hydroxy-tetrazole group is transformed into an alkyne by EDC (a water-soluble crosslinker) treatment. The second alkyne, called latent alkyne (2), is used for a second CuAAC reaction (3), allowing the addition of a second molecule.
The second alkyne being latent (not in the form of an alkyne during the first ligation) allows the grafting of a different molecule on the latter. This process can be seen as an orthogonal use of click chemistry.
Development stage
Various compounds already synthesized
Benefits
- Multiple reactivity of the alpha-hydroxy-beta tetrazole group
- Reaction at ambient temperature
Industrial applications
- Bioconjugation (ligation of chromophores on biomolecules)
- Photovoltaics (grafting of active compounds on surfaces)
- Medicinal chemistry (automated preparation of peptidomimtics on surfaces, thanks to the 1,4-triazole which is a peptidic bond analog)