Process for phenols synthesis
Référence
00755-06
Mots-clés
Statut des brevets
French patent application FR0902767 filed on June 8, June 2009 entitled « Procédé de synthèse de phénols »


Inventeurs
Marc TAILLEFER
Ning XIA
Florian MONNIER
Anis TLILI
Statut commercial
Exclusive or non-exclusive licence.
R&D Partnership
Laboratoire
Institut Charles Gerhardt – Institut de chimie moléculaire et des Matériaux, (UMR5223) in Montpellier, France.
Description
CONTEXT
Phenols are very important intermediates in the chemical, pharmaceutical and materials industry. More than 7.2 megatons of phenol itself are produced per year, which is one of the most important chemicals for industry. Nowadays about 90% of the world’s phenol demand is being satisfied by the Hock process which corresponds to peroxidation of cumene, itself obtained from benzene propylation. This industrial process, which is not very efficient in yield (overall 5%) and energy consuming, also produces large amounts of co-products. Alternative oxidation technologies that avoid production of acetone have been proposed but none have succeeded in replacing the Hock process.
For the preparation of functionalized phenols, non-oxidative methods such as traditional nucleophilic aromatic substitution are used; however the range of substituents is often limited by the requisite harsh reaction conditions or by the electronic requirements of the substrate. An Iridium based catalytic system for preparation of non-ortho-substituted phenols involving a one-pot aromatic borylation/oxidation sequence has been reported. Recently, have been developed systems based on palladium/phosphine ligands catalysis allowing the selective formation of phenols from different aryl halides. However, these systems are more expensive than copper and not very favorable for toxicity issues.
So the development of a cheaper copper catalyzed system enabling the direct hydroxylation of aryl halides has become an important goal. There are however two critical problems to be resolved: first, the direct copper catalyzed coupling reaction between unactivated aryl halides and hydroxide has never been reported below 200°C. One study showed that this reaction is very difficult even at 200- 300°C with microwave heating. Secondly, copper catalyzed coupling between phenols and aryl halides is well known. Hence, this reaction could be an important concurrent reaction once phenol is formed in situ, so only symmetric diarylethers might be produced.
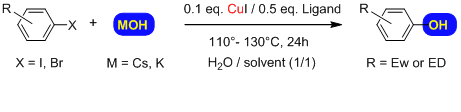
We here report that the selective hydroxylation of both activated and unactivated aryl bromides and iodides can be achieved by a very simple aqueous solution using hydroxide as the base in a catalytic system in which low toxicity and inexpensive copper is used together with simple bidentate ligands.
TECHNICAL DESCRIPTION
Copper catalyzed hydroxylation of activated and inactivated aryl and heteroaryl iodides or bromides is achieved using metal hydroxide salts.
BENEFITS
We have discovered a general, economical and efficient method for the direct copper-catalyzed synthesis of phenols from aryl iodides and bromides. This selective procedure avoids the classical formation of the related biaryl ether by-product. The H2O/co-solvent system used is unusual and work is currently in progress to elucidate how it functions. The convenience of aqueous hydroxide anion and the low cost of the copper catalytic system make this method very competitive. It could be easily adaptable to industrial scale production, especially where safety and environmental factors are of greater concern. The catalytic system is not that different from that which allowed us in 2007 to perform the Cu-catalyzed synthesis of anilines using aqueous ammonia.[see patent WO2009050366]. With these tools in hand we can now propose a general alternative, both for amination and hydroxylation of aromatic halides, to the existing Pd-based catalytic systems.
PUBLICATIONS
A Very Simple Copper-Catalyzed Synthesis of Phenols by Employing Hydroxide salts
A. Tlili, N. Xia, F. Monnier, M. Taillefer
Angewandte Chemie, 2009
For further information, please contact us (Ref 00755-06)