METHOD FOR MANUFACTURING A MINIATURIZED ELECTROCHEMICAL CELL
Référence
06616-01
Mots-clés
Miniaturization, electrochemical cells, electrodes, biofuel cells.
Statut des brevets
European priority patent application n° EP14306341 filed on August 29, 2014 and entitled "Method for manufcaturing a miniaturized electrochemical cell"

Inventeurs
Nicolas MANO
Alexander KUHN
Serge RAVAINE
Matthias HEIM
Stéphane RECULUSA
Statut commercial
Exclusive or non-exclusive license, collaborative research agreement
Laboratoire
Centre de Recherche Paul Pascal (CRPP, UPR8641), Pessac, France
NSYSA (UMR5255), Pessac, France
Description
TECHNICAL DESCRIPTION
The invention relates to a method for manufacturing a miniaturized electrochemical cell. This invention addresses this need by using a colloidal template for manufacturing miniaturized electrochemical cells consisting only of electrodes with a high active surface area, i.e. macroporous electrodes.
DEVELOPMENT STAGE
We report the bottom-up design of a fully integrated miniaturized electrochemical cell consisting of two independently addressable macroporous electrodes with well-defined porosity, controllable thickness and easily tunable spacing. The concept is based on the electrodeposition of alternating gold-nickel-gold metal layers through a colloidal crystal synthesized by the Langmuir-Blodgett technique. The proposed architecture shows good structural stability and a significant increase of active surface area.
We demonstrate that electroactive species react efficiently with both electrodes, thus opening up new applications in the field of miniaturized electrochemical devices due to the versatility of this straightforward approach.
BENEFITS
The presented structure is the first example of a fully integrated miniaturized electrochemical cell with performance-enhancing macroporous electrodes. Such architectures with a versatile design principle are potentially suitable for the elaboration of implantable miniaturized (bio)fuel cells and/or biosensors. They combine an artificially increased active surface area with an improved mass transport due to the radial diffusion in the case of a cell with coaxial geometry. These characteristics play an essential role and ensure higher current densities and power output.
INDUSTRIAL APPLICATIONS
Any electrochemical devices requiring miniaturization and high specific surface area electrodes, especially for applications in the field of energy storage devices such as batteries, supercapacitors, fuel cells and biofuel cells.
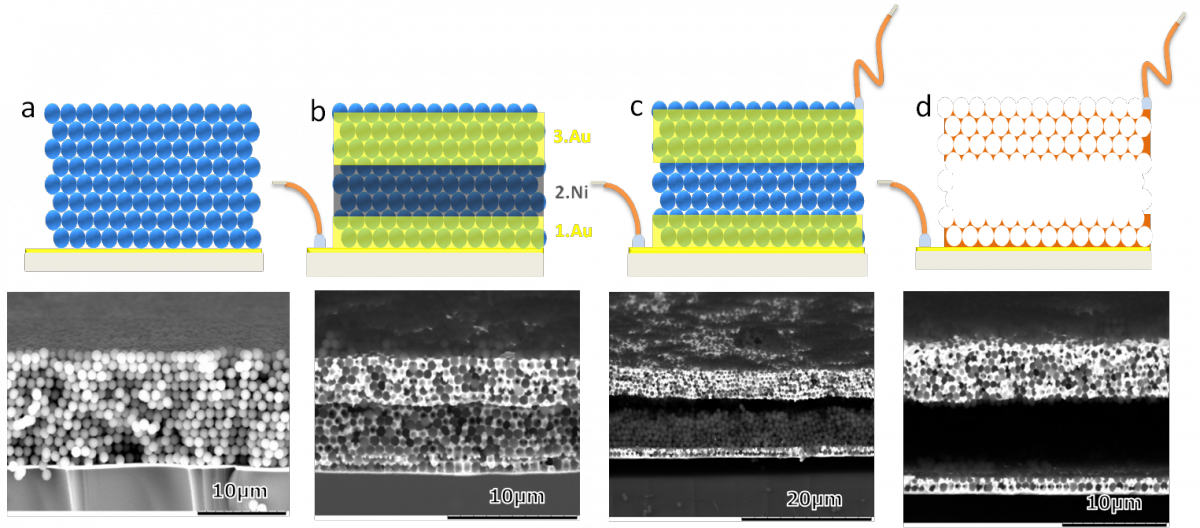
Schematic representation showing the different steps of the fabrication of the miniaturized two electrode electrochemical cell (first row) with the corresponding cross sectional SEM images (second row). a) Assembly of the colloidal crystal template made of silica beads (1 µm diameter), b) electrodeposition of a gold-nickel-gold layer in a template obtained with 740 nm silica beads c) selective dissolution of the sacrificial nickel layer d) final two electrode device with controlled electrode spacing in the micrometer range obtained after dissolution of the silica template.
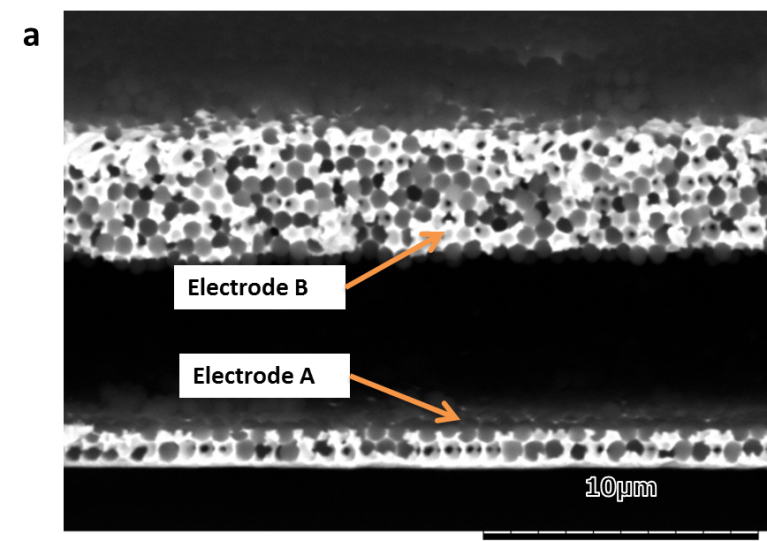
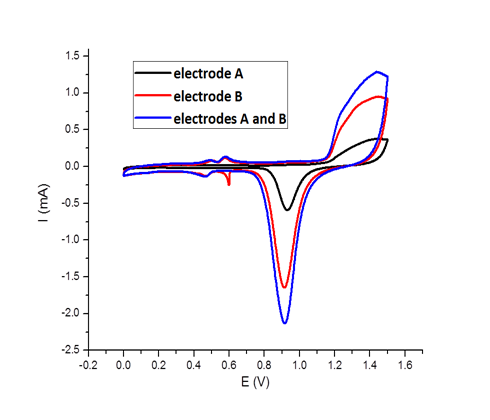
a) Cross sectional SEM picture showing a porous structure after the dissolution of nickel with sulfuric acid b) CV stripping curves (recorded at 25 °C, scan rate: 100 mV/s in 0.5M sulfuric acid solution) of macroporous gold electrodes deposited on a single support and separated by an electrolyte gap. Electrode A corresponds to the lower electrode (3 half-layers of macroporous gold) and electrode B to the upper one (10 half – layers macro porous gold).
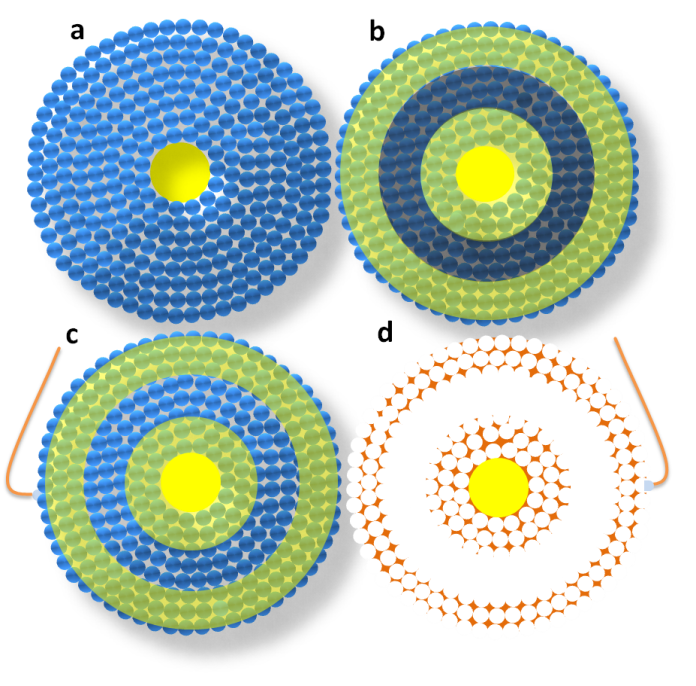
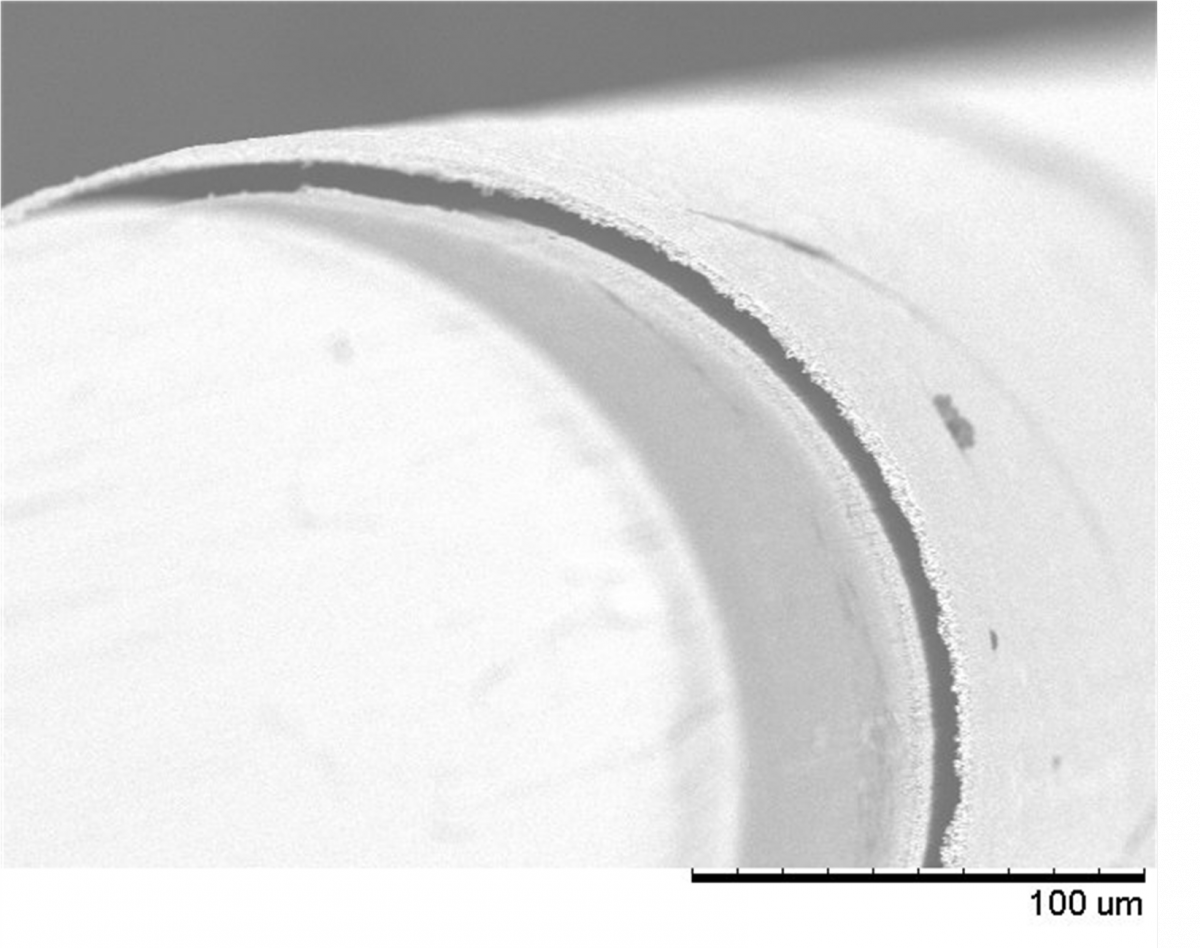

Schematic representation in cross-sectional view of the key steps for the elaboration of a miniaturized coaxial two-electrode electrochemical cell. Steps a, b, c and d are identical to those in Figure 1, with the difference that the initial substrate is a gold microwire (yellow disk in the middle) instead of a flat surface.
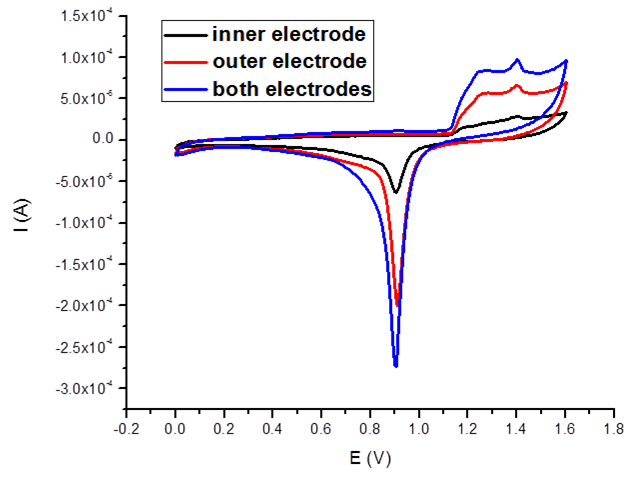
Electrochemical characterization of structural stability and electrical independence of the coaxial electrodes once the silica beads are removed from the structure. The corresponding active surfaces of the independent coaxial electrodes and of the short-circuited system were 0.26 cm2, 0.62 cm2 and 0.93 cm2 respectively. CVs have been recorded at ambient temperature, at a scan rate of 100 mV/s in 0.5M sulfuric acid solution.
For further information, please contact us (Ref 06616-01)