New cellular and animal toxic model of Parkinson’s disease
Référence
05155-01
Mots-clés
Parkinson’s disease, Cellular model, Animal model, Toxic model
Statut des brevets
Priority patent application FR1260979 filed on November 19th, 2012 entitled » Modèle chimique d’une maladie neurodégénérative, procédé de préparation et utilisations »


Inventeurs
Thierry MARTENS
Christophe MORIN
Michaël RIVARD
Céline LAURENCE
Sonia LEHRI-BOUFALA
Statut commercial
Exclusive or non-exclusive licenses, Collaborative agreement
Laboratoire
Institut de Chimie et des Matériaux de Paris Est (ICMPE), a CNRS – Université Paris-Est Créteil laboratory (UMR7182) in Vitry, France.
Description
CONTEXT
Parkinson’s disease (PD) is the second most common neurodegenerative disease, affecting 1% of the population over 55 years of age. The main features of PD are tremor, rigidity, bradykinesia, and postural instability; however, these motor manifestations can be accompanied by nonmotor symptoms such as olfactory deficits, sleep impairments, and neuropsychiatric disorders. This disease is characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta (SNc), a profound deprivation of dopamine (DA) in the striatum, and the presence of intracytoplasmic inclusions called Lewy bodies (LB), which are composed mainly of α-synuclein and ubiquitin.
Breakthroughs in the last two decades using cellular and animal models have helped to identify specific events of Parkinson’s Disease. Most popular models include those produced by 6- hydroxydopamine (6-OHDA), 1-methyl-1,2,3,6-tetrahydropiridine (MPTP), rotenone, and paraquat, as well as several genetic animal models like those related to alpha-synuclein, PINK1, Parkin and LRRK2 alterations.
A common feature of all toxin-induced models is their ability to produce an oxidative stress and to cause cell death in DA neuronal populations that reflect what is seen in PD. However, the timing of the disease progression in these models is much faster than in the human condition. Besides, while no consistent Lewy Bodies-like inclusion formation is observed. A model closer to the human disease is needed.
TECHNICAL DESCRIPTION
This new model of Parkinson Disease is induced by a new and easy-to-synthesized chemical compound: pyridinium furosemide. Our data shows that the hallmarks of PD (α-synuclein aggregation, caspases activation, inhibition of mitochondrial complex I) appear in a much slower manner than with MTPT or MPP+: cell death in SH-SY5Y cells after an exposition to the compound during 96h is equivalent to that observed after a 6h-incubation with MPP+.
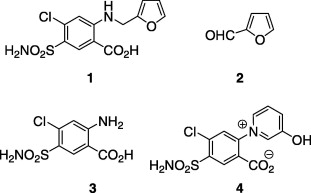
Pyridinium furosemide
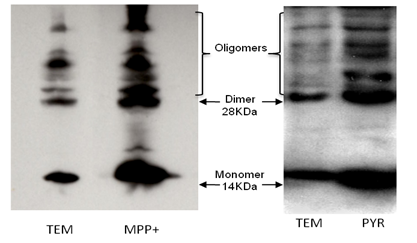
α-synuclein aggregation in SH-SY5Y cells exposed 24h to 0.5 mM MPP+ or 96h to furosemide pyridinium 1mM)
BENEFITS
The slower rate of appearance of the hallmarks of PD compared to the other models should enhance the research on the very first steps of the disease.
This tool may be used for the screening of new drugs or the research for new biomarkers.
Importantly, pyridinium furosemide is a degradation product of furosemide, a drug which has been widely used as a diuretic since the nineteen sixties. It is one of the forty compounds having the highest risk with a predicted environmental concentration greater than 100 ng/L.
INDUSTRIAL APPLICATIONS
In vitro and in vivo screening of PD therapeutic strategy.
DEVELOPMENT STAGE
Cellular model is standardized and ready to use
Preliminary data in rats and in mice show that the treatment of an animal with this compound also induces symptoms of early stages of Parkinson Disease: α-synuclein accumulation in the striatum, mitochondrial respiratory chain inhibition, REM sleep troubles, etc…
PUBLICATIONS
Anticipating the fate and impact of organic environmental contaminants: A new approach applied to the pharmaceutical furosemide.
Laurencé C. Chemosphere, Volume 113, 2014, 193 – 199
Preparative access to transformation products (TPs) of furosemide: a versatile application of anodic oxidation.
Laurencé C. Tetrahedron, 67 (2011), pp. 9518–9521
For further information, please contact us (Ref 05155-01)
Besoin de plus d'informations ?
Nous contacterTechnologies Liées
-
24.02.2015
High Throughput Screening of LINGO Ligands: A New Drug Discovery Tool for the Treatment of Neurodegenerative Diseases
Outils de recherche et criblage 03247-01
-
11.02.2014
The frequency of cellular nodules as a new marker for metastase in colorectal cancer
Outils de recherche et criblage 85349-01
-
04.10.2013
Method for screening anticancer substances, set or kit for implementing said method
Outils de recherche et criblage 86825-01